Lipid nanoparticles (LNPs) are a class of nanocarriers that have gained significant attention in recent years due to their ability to effectively deliver various therapeutic molecules, including mRNA, siRNA, and small molecules. These particles are composed of lipid-based components, such as phospholipids, cholesterol, and helper lipids, which self-assemble into spherical structures.
Key Advantages of LNPs:
- Enhanced Delivery: LNPs can significantly improve the bioavailability and efficacy of therapeutic molecules by protecting them from degradation and facilitating their uptake by target cells.
- Targeted Delivery: By incorporating specific ligands or targeting moieties, LNPs can be designed to deliver therapeutic molecules to specific tissues or cell types, reducing off-target effects.
- Versatility: LNPs can be used to deliver a wide range of therapeutic molecules, including mRNA, siRNA, and small molecules.
- Safety: LNPs have been shown to have a generally favorable safety profile, with minimal toxicity.
Applications of LNPs:
- mRNA Vaccines: LNPs have played a crucial role in the development of mRNA-based COVID-19 vaccines, which have been highly effective in preventing the spread of the virus.
- Gene Therapy: LNPs can be used to deliver therapeutic genes to treat genetic disorders, such as cystic fibrosis and hemophilia.
- RNA Interference (RNAi): LNPs can be used to deliver siRNA molecules to silence specific genes, which has potential applications in the treatment of various diseases, including cancer and viral infections.
- Drug Delivery: LNPs can be used to deliver small molecule drugs, such as chemotherapeutic agents, to target cells in a more efficient and less toxic manner.
Challenges and Future Directions:
- Manufacturing: The large-scale production of LNPs can be challenging, and efforts are ongoing to develop more efficient and cost-effective manufacturing processes.
- Immunogenicity: In some cases, LNPs can induce an immune response, which may limit their efficacy or cause adverse effects.
- Stability: LNPs can be sensitive to storage conditions, and efforts are being made to develop more stable formulations.
LNPs remain a promising delivery system with the potential to revolutionize the treatment of a wide range of diseases. Ongoing research and development are focused on addressing the limitations of LNPs and expanding their applications.
Oct 16, 2024
Research Title: “In situ engineering of mRNA-CAR T cells using spleen-targeted ionizable lipid nanoparticles to eliminate cancer cells”
(Source) December 2024
Lipid nanoparticles (LNPs) and CAR T cell therapy: just a simple crossover or a leap forward in solid tumor treatment?
In recent years, CAR T cell therapy has revolutionized the treatment of B-cell lymphoma, but its effectiveness against solid tumors remains limited -for instance, most CAR T cells are transduced using viral vectors, which may introduce unknown risks and safety concerns related to mutagenic integration and inflammatory toxicity.
Now, this new study introduces a “potential” breakthrough: ionizable LNPs that deliver mRNA to engineer T cells in vivo, bypassing traditional manufacturing hurdles.
🔬 This approach uses anti-CD3 antibody-armed LNPs to precisely deliver mRNA encoding CARs, enabling rapid production of functional CAR T cells directly within the body. These cells target melanoma with high TRP1 expression, infiltrating tumors and curbing progression.
🌟 A key improvement?
Including IL-7, which boosts T cell proliferation and activity, while combining it with anti-PD-1 antibodies prevents exhaustion, increasing the overall antitumor potency without triggering severe side effects like cytokine release syndrome (CRS).
The Graphical Abstract is given below. It is taken from the same research article:
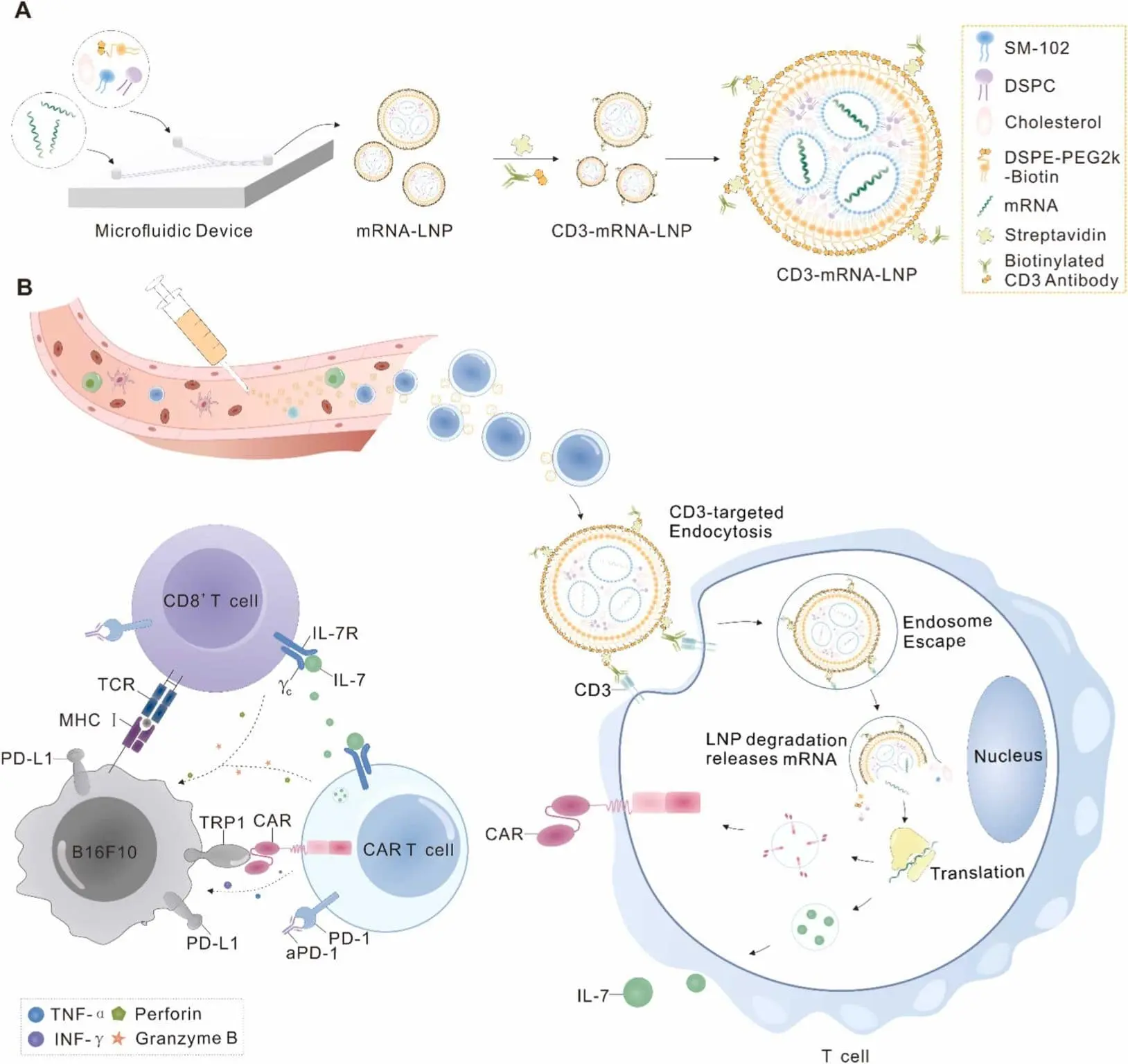
💡 What makes this approach cool?
It offers a cost-effective, scalable, and safer method of generating CAR T cells, eliminating the need for ex vivo processing and lymphodepleting chemotherapy. The potential applications extend beyond melanoma, with opportunities for treating other cancers and diseases.
Other References:
- “Steering the course of CAR T cell therapy with lipid nanoparticles” Link
- “Lipid nanoparticles outperform electroporation in mRNA-based CAR T cell engineering” Link
- “Critical considerations of mRNA–LNP technology for CAR-T therapy: components, payloads and emerging horizons” Link
Oct 10, 2024
Research Title: “Discovery of Ketal-Ester Ionizable Lipid Nanoparticle with Reduced Hepatotoxicity, Enhanced Spleen Tropism for mRNA Vaccine Delivery”
(Source) October 2024
(4S)-KEL12: A safer and more efficient LNP for mRNA vaccine delivery?
Safe and effective delivery systems are essential for the performance of LNP-based RNA therapeutics. Currently, only 3x ionizable amino lipids are clinically approved for RNA therapies: DLin-MC3-DMA (used in siRNA therapy to knock down transthyretin in hepatocytes) plus ALC-0315 and SM-102, structurally similar lipids used in BioNTech and Moderna SARS-CoV-2 mRNA vaccines.
Despite their usefulness, drawbacks arise, such as mild to moderated local and systemic adverse events (for DLin-MC3-DMA), which call for developing novel lipids. Now, a team has developed a novel LNP system using (4S)-KEL12, a ketal ester lipid designed for enhanced safety and efficacy in mRNA therapies.
By incorporating multiple biodegradable ketal and ester groups into the lipid structure, the authors aimed to improve biocompatibility and reduce toxicity compared to ionizable lipids like DLin-MC3-DMA and SM-102. Through iterative optimization of the lipid’s head and tail groups, they fine-tuned its pKa and molecular shape, identifying (4S)-KEL12 as a promising candidate.
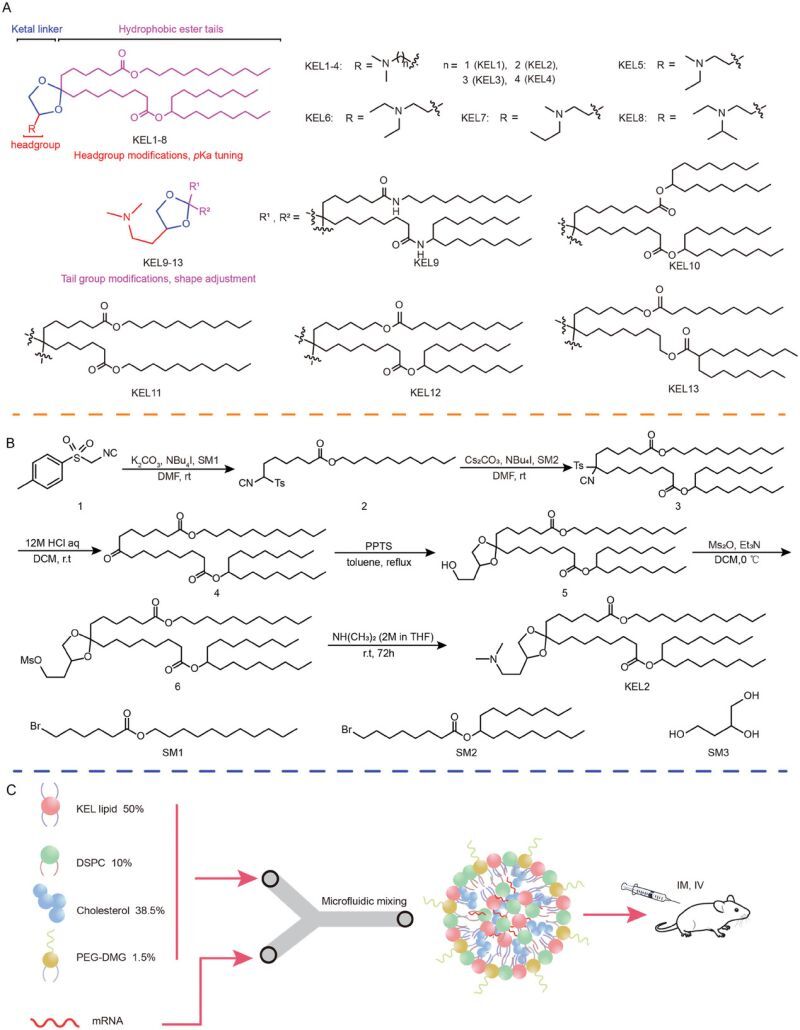
The studies showed that (4S)-KEL12 LNPs exhibit significantly higher delivery efficacy and lower toxicity than DLin-MC3-DMA LNPs. Notably, compared to SM-102 LNPs, they demonstrated better spleen tropism and reduced liver accumulation, which is crucial for minimizing hepatotoxicity. Additionally, (4S)-KEL12 demonstrated good biodegradability, as it was undetectable in major organs (heart, liver, lung, kidney) 120 hours after IM injection and undetectable in the blood 24 hours after IV injection.
When used to deliver a therapeutic mRNA cancer vaccine encoding HPV antigens, (4S)-KEL12 LNPs elicited robust cellular immune responses. This led to substantial tumor regression and prolonged survival in tumor-bearing mice, highlighting their potential in effective cancer immunotherapies.
Comprehensive analyses—including PK, organ distribution, biodegradability, and cellular expression profiles—provided valuable insights into the favorable immune cell targeting and reduced side effects of the LNPs.
Oct 03, 2024
Research Title: “Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d”
(Source) October 2024
💡 Breaking new ground in RNA therapeutics with LNPs: are amine headgroups in ionizable lipids driving immune response?
LNPs are undoubtedly the gold standard for RNA delivery, but their immunogenic properties were not fully understood until now. Recent findings shed light on ionizable lipid chemistry’s key role in driving immune responses, offering vital insights for enhancing LNP safety and efficacy.
🔬 The study reveals that amine headgroups in ionizable lipids trigger innate and adaptive immunity by interacting with Toll-like receptor 4 (TLR4) and CD1d and promoting lipid-raft formation. These interactions skew the immune system toward a type-1 T-helper response, increasing pro-inflammatory cytokines and IgG2c/IgG1 levels.
A combined image is given below. It is taken from the same research article:
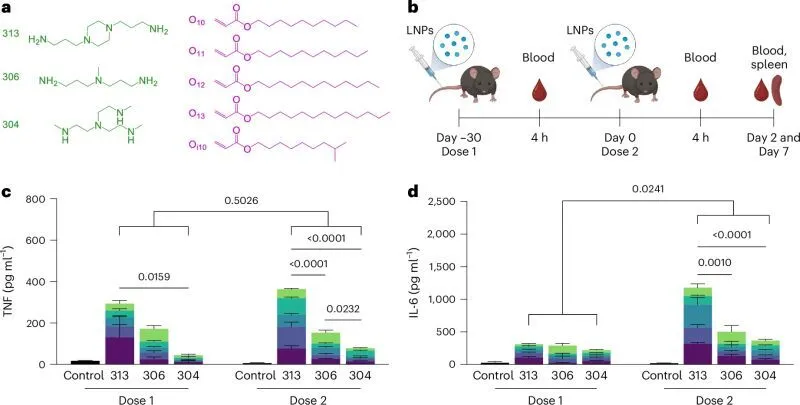
Since TLR4 and CD1d are expressed on the cell and endosomal membranes of immune cells, it is likely that immune stimulation occurs at both sites. Lipid receptor binding seems to prompt the release of pro-inflammatory cytokines from innate and adaptive immune cells, reducing the concentration of anti-PEG IgM antibodies available to bind LNPs, thus preserving LNP efficacy upon repeat dosing.
🌟 Moreover, the authors provided a way to use computational methods to predict LNP-induced immune responses based on lipid structure, a powerful tool that will help fast-track the development of safer, well-tolerated LNPs for RNA-based therapies.
Oct 01, 2024
Research Title: “Combinatorial design of siloxane-incorporated lipid nanoparticles augments intracellular processing for tissue-specific mRNA therapeutic delivery”
(Source) October 2024
💡 Siloxane-LNPs, or how to significantly augment intracellular processing of nanoparticles for tissue-specific mRNA therapeutic delivery
Researchers have engineered siloxane-incorporated LNPs (SiLNPs) that offer precise, organ-specific targeting of mRNA, opening doors to more effective treatments for gene editing, protein replacement, and regenerative medicine.
🔬 Owing to the high stability, low chemical reactivity and good biocompatibility of siloxane moieties, the authors have integrated them into ionizable lipids forming SiLNPs that can enhance cellular uptake and endosomal escape, boosting mRNA delivery efficiency.
Upon in vivo evaluation, the authors observed that minor structural alterations of siloxane-incorporated lipidoids can substantially alter organ tropism. For instance, liver-, lung- and spleen-targeted SiLNPs show organ-specific transfection of various cell types, including hepatocytes, Kupffer cells, endothelial cells (ECs), dendritic cells, and splenic macrophages in an Ai14 mouse model.
Moreover, using organ-specific SiLNPs to deliver CRISPR–Cas9-based gene editors, robust gene knockout in the liver of wild-type mice and in the lungs of transgenic GFP and Lewis lung carcinoma (LLC) tumor-bearing mice was achieved. Remarkably, these lung-targeted SiLNPs were also able to repair lung damage caused by viral infection through the delivery of angiogenic factors, showcasing their therapeutic potential.
This study supposes a proof-of-concept demonstration that tuning in vivo LNP organ targeting can be achieved by incorporating a siloxane moiety into the lipidoid structure, enabling the development of next-generation lipid-like materials for tissue-specific mRNA delivery.
Sept 27, 2024
Research Title: “In Silico Engineering of Stable siRNA Lipid Nanoparticles: Exploring the Impact of Ionizable Lipid Concentrations for Enhanced Formulation Stability”
(Source) September 2024
🔬 Exploring the stability in siRNA LNPs for RNA-based therapeutics
In the ever-evolving field of gene therapy, LNPs are critical for the delivery of RNA-based drugs like siRNA and mRNA. A recent study (currently as a pre-print), leveraging molecular dynamics simulations, dives into the formulation stability of siRNA-loaded LNPs, focusing on ionizable lipid concentrations.
Key findings revealed that LNPs containing positively charged ionizable lipids significantly enhance siRNA encapsulation, offering over double the efficiency of neutral lipids. These positive lipids create stronger interactions with siRNA, which also impacts the force required for siRNA to escape the nanoparticle—a key factor in ensuring stable and effective drug delivery.
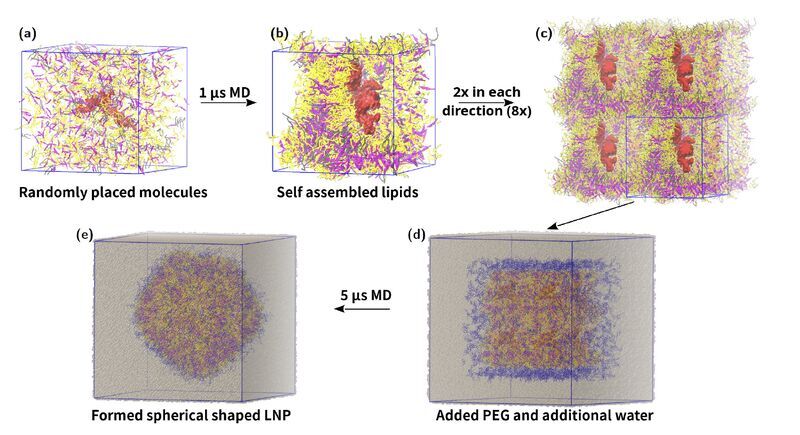
The study also highlights the challenges with neutral lipids at higher concentrations, where aggregation can occur, leading to potential instability. Their work, however, was limited to the stability of siRNAs inside LNP with various lipid compositions. Moreover, endosomal escape was not discussed since it falls outside the scope of the investigation.
Overall, these insights underscore the importance of carefully optimizing LNP formulations to balance stability and delivery efficacy, which is essential for the future of RNA therapeutics. On the other hand, this computational approach paves the way for designing more robust delivery platforms that can be adapted for a variety of RNA-based treatments, from gene silencing to mRNA vaccines.